Summary
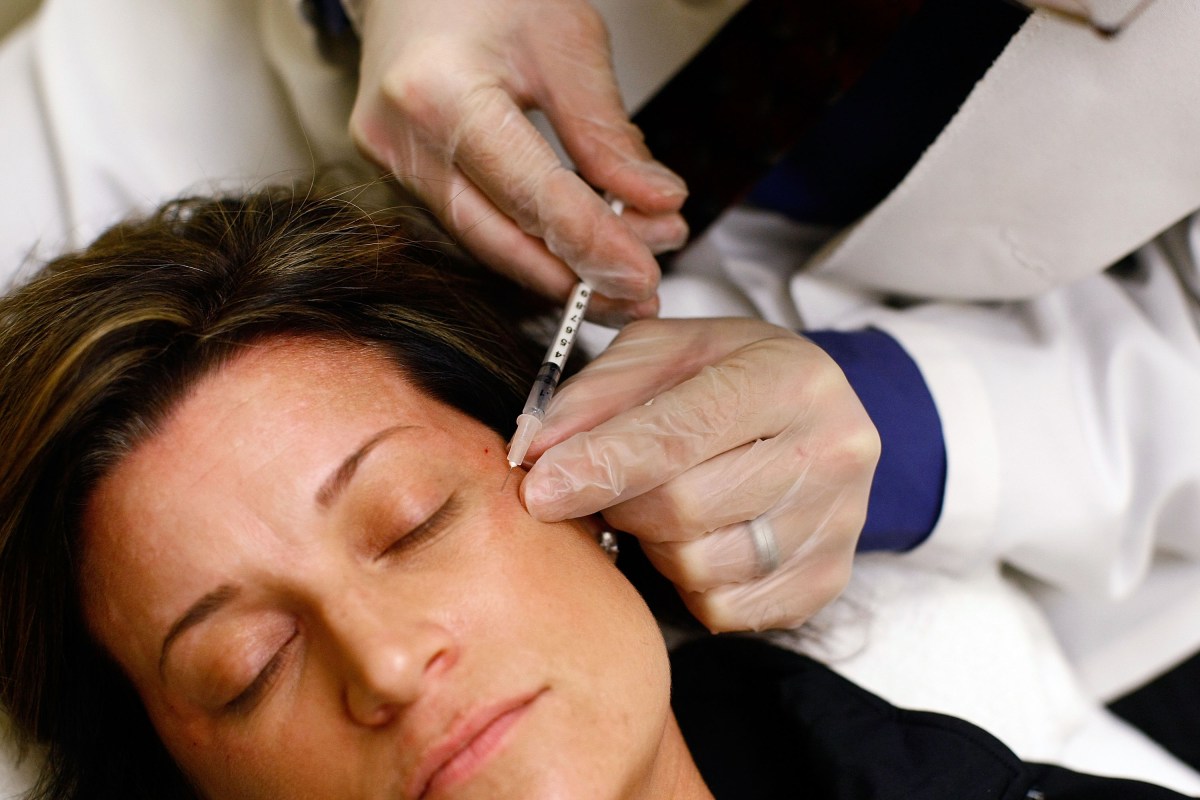
The FDA has warned companies against selling unapproved Botox products because they are linked to a rare but serious illness called botulism. The agency has sent warning letters to 18 websites selling these potentially dangerous products, which have caused illnesses in at least 22 individuals.
Key Facts
- The FDA issued warnings to 18 websites for selling unapproved Botox.
- Unapproved Botox can cause botulism, a serious illness that can be deadly.
- Botulism symptoms include muscle weakness, trouble swallowing, and breathing difficulties.
- 22 people reported botulism-related illnesses in 11 states from November 2023 to March 2024.
- The illnesses mainly affected women aged 25 to 59 who received injections outside licensed facilities.
- 11 individuals required hospitalization due to the conditions.
- The FDA advises consumers to use only FDA-approved Botox products and seek treatment from licensed professionals.
- If symptoms of botulism appear after an injection, immediate medical attention is recommended.