Summary
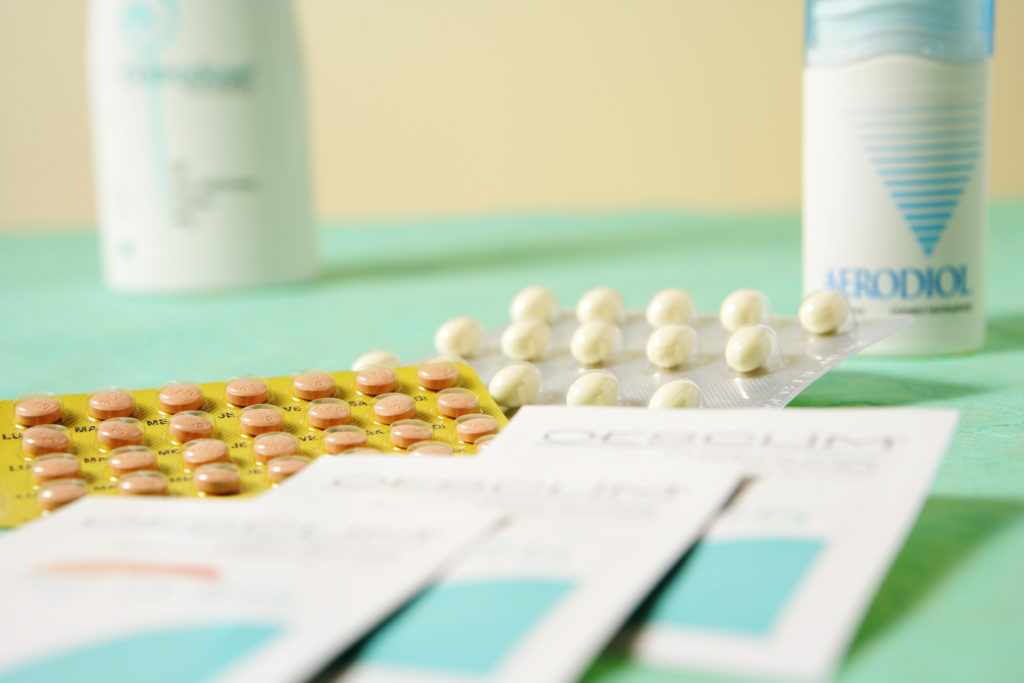
The U.S. Food and Drug Administration (FDA) announced it will remove most "black box" safety warnings from hormone drugs used to treat menopause symptoms. This change follows advocacy from medical professionals and new research suggesting these drugs are safer than previously believed, specifically for women under 60.
Key Facts
- The FDA decided to remove most "black box" warnings from menopause hormone therapy drugs on November 10.
- "Black box" warnings indicate serious health risks on medication labels.
- These warnings were first required in 2003 after a study suggested increased risks of breast cancer, stroke, and heart attack.
- Recent studies show these medications are safer than previously thought, especially for women younger than 60.
- Hormone therapy drugs treat menopause symptoms like hot flashes and mood swings.
- There are two main types of hormone therapies: local and systemic.
- The FDA will keep black box warnings for endometrial cancer on estrogen-only systemic medications.
- The choice of therapy highly depends on individual patient needs, as emphasized by experts.