Summary
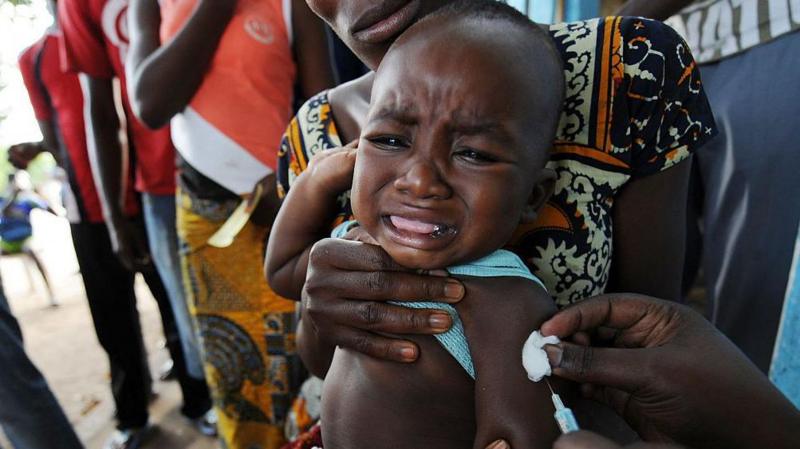
The World Health Organization (WHO) criticized a planned U.S.-funded vaccine trial in Guinea-Bissau as unethical. The study aimed to compare the effects of giving a hepatitis B vaccine at birth versus at six weeks old to newborns. Due to public backlash and WHO's concerns, the Guinea-Bissau government suspended the trial.
Key Facts
- The trial was funded by the U.S. Centers for Disease Control and Prevention and involved about 14,000 babies.
- WHO called the trial unethical and expressed concerns about scientific and ethical standards.
- WHO emphasized that the hepatitis B vaccine is a proven life-saving intervention.
- Guinea-Bissau planned to introduce the birth-dose vaccine by 2028, aligning with WHO recommendations.
- More than 12% of Guinea-Bissau's adult population has chronic hepatitis B.
- Public outrage in Guinea-Bissau led to the suspension of the study.
- Critics, including Guinea-Bissau's former health minister, opposed treating local babies as test subjects.
- The U.S. health department involved in the trial was led by Robert F Kennedy Jr., known for questioning vaccines.